This page deals with the addition of the alkaline solutions ammonium hydroxide to solutions containing metal ions with 2+ and a 3+ charge. To fully understand the ideas covered here it is important that you are very familiar with the acidity or hydrolysis reactions that take place in aqueous solutions containing metal ions, click the link above to review these acidity reactions if you need to. It is also worth quickly reviewing the page on precipitation reactions using sodium hydroxide and solutions of metal ions.

Ammonia (NH3) is a highly soluble gas which dissolves in water to form the weak alkali ammonium hydroxide, this solution is alkaline due the presence of the hydroxide ion (OH-) as shown in the equation below:
In dilute solutions of ammonia the concentration of ammonia (NH3) present will be larger than the concentrations of hydroxide ions (OH-) and ammonium ions (NH4+) simply because ammonium hydroxide is a weak alkali so the position of equilibrium in the above equation will lie to the left.
Now we already know that when an alkaline solution such as sodium hydroxide is added to a solution containing a transition metal ion that a solid precipitate of the metal hydroxide is formed. It should come then as no surprise that when a solution of ammonia is added to a solution containing a transition metal ion that initially the same precipitation reaction occurs. However a there is a complication present here due to the fact that ammonia can not only act as a base and cause these precipitation reactions to happen but ammonia is also capable of acting as a ligand, which results in a ligand substitution or ligand exchange reaction taking place. However these ligand substitution reactions only happen if an excess or a concentrated solution of ammonia is used.
Now recall from the acidity or hydrolysis reactions that takes place when a transition metal salt is dissolved in water that the main species present in the equilibrium mixture that forms will be the hexaaqua ion containing the transition metal ion, for example the equation below shows the first reversible reaction that takes place when a transition metal salt containing a transition metal ion with a 2+ charge is dissolved in water.
Now the main species present in this equilibrium mixture will be the hexaaqua ion - M(H2O)6]2+ since the position of equilibrium lies very much to the left hand side. However addition of a base such as sodium hydroxide will result one or more of the acidic water ligands present in the hexaaqua complex to be deprotonated, that is to lose a hydrogen ion (H+). If one hydrogen ion (H+) is lost this will form:
And if more sodium hydroxide is added then one more acidic hydrogen ion (H+) will be removed from a water ligand in the [Cu(H2O)5(OH)]+ complex to form the insoluble precipitate of copper hydroxide:
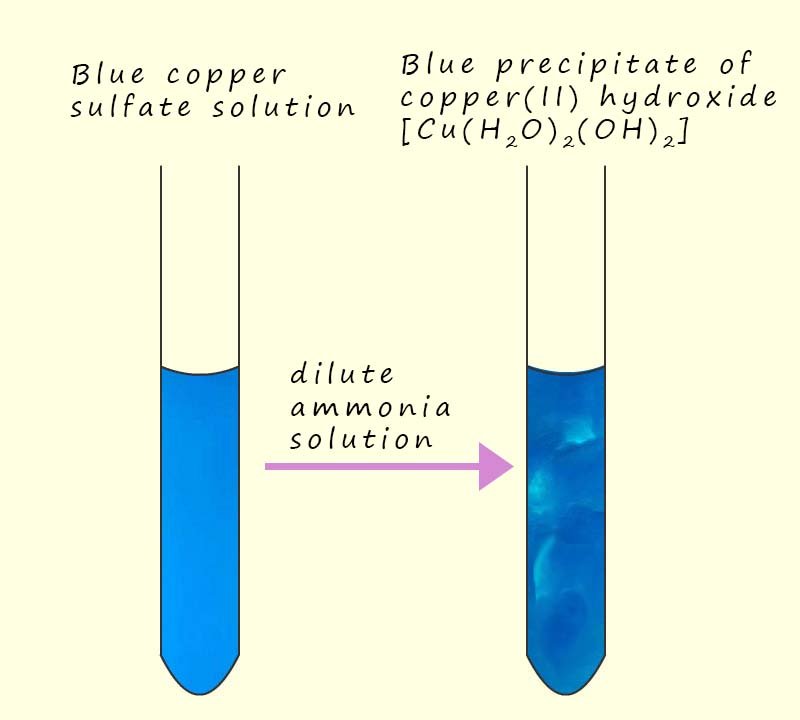
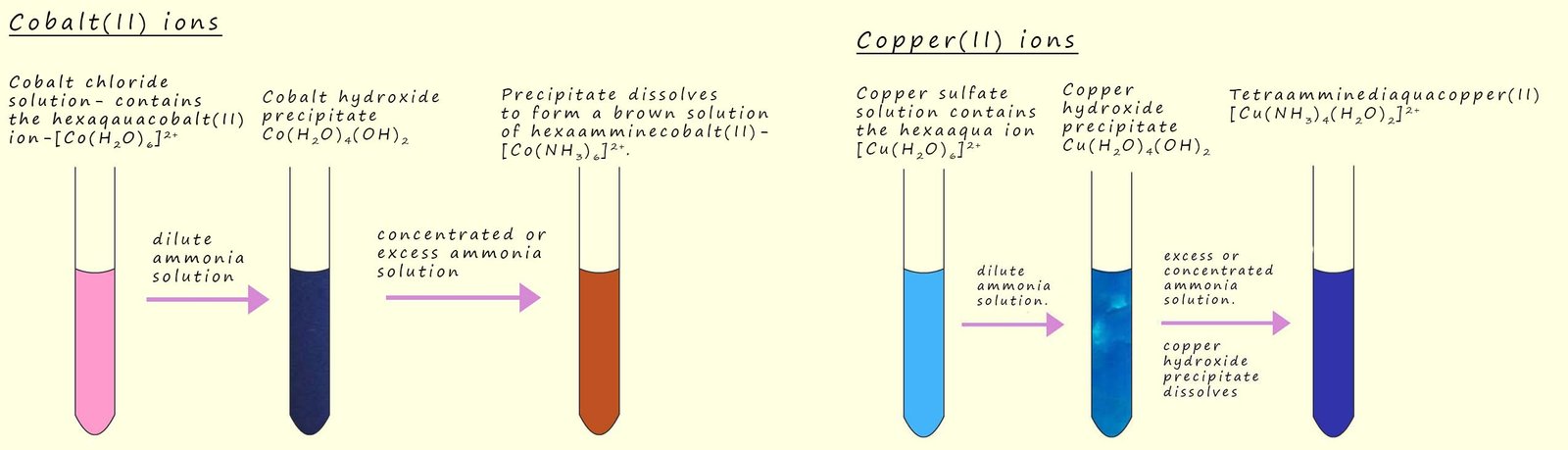
A blue copper (II) sulfate solution will produce a blue precipitate of copper (II) hydroxide when a small amount of a dilute ammonia solution is added, this is shown in the image opposite.
Since the main species present in the acidity reaction of a hexaaquacopper(II) salt with water is the hexaaqua complex [(Cu(H20)6)]2+, it would seem reasonably then that when a small amount of dilute ammonia is added the basic ammonia will simply remove two hydrogen ions (H+) from the acidic water ligands attached to the hexaaqua complex until a precipitate of the solid copper (II) hydroxide is formed. Each of the hydrogen ions (H+) will be remove in a step-wise manner, one after the other. We can show this as:
And for the removal of a second hydrogen ion (H+) we have:
With the loss of a second hydrogen ion (H+) the complex formed has no charge, that is it is neutral and so will be insoluble and will form a solid precipitate. We can of course simply combine the two equations above to give:
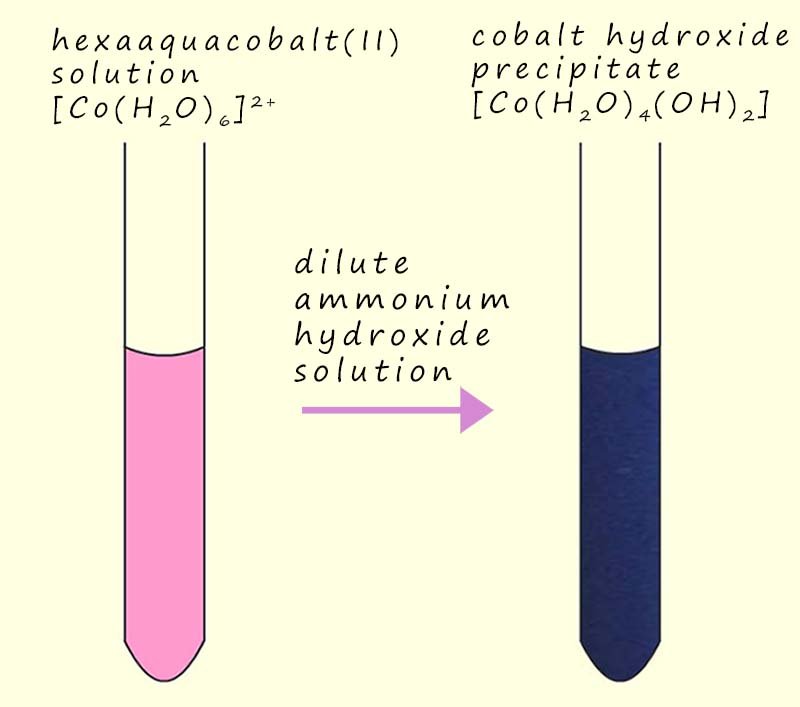
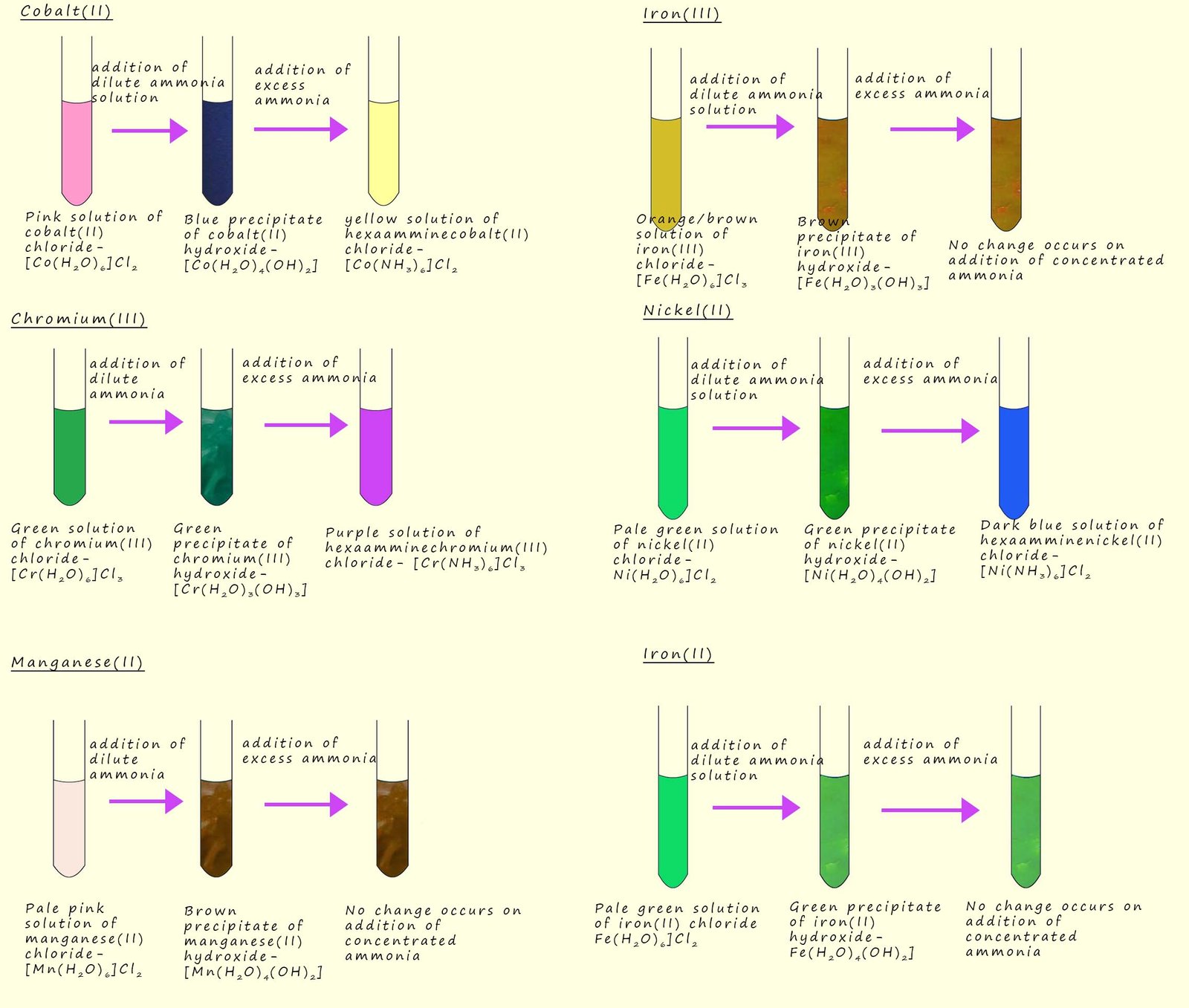
The addition of a dilute ammonia solution to many hexaaqua transition metal salts will result in the formation of many different coloured precipitates of the metal hydroxide, for example when a few drops of dilute ammonia solution are added to a pink hexaaquacobalt(II) solution a blue precipitate of cobalt (II) hydroxide is produced, this is shown in the image opposite and an equation for this precipitation reaction is shown below:
Both the examples given above involve metal ions with a 2+ charge, however if we swap these for a metal ion with a 3+ charge the reaction would be very similar, the only difference is that three hydrogen ions (H+) would have to be removed by the basic ammonia before the insoluble neutral metal hydroxide would be formed as a precipitate, we can show the reaction for a 3+ metal ion with ammonia as:
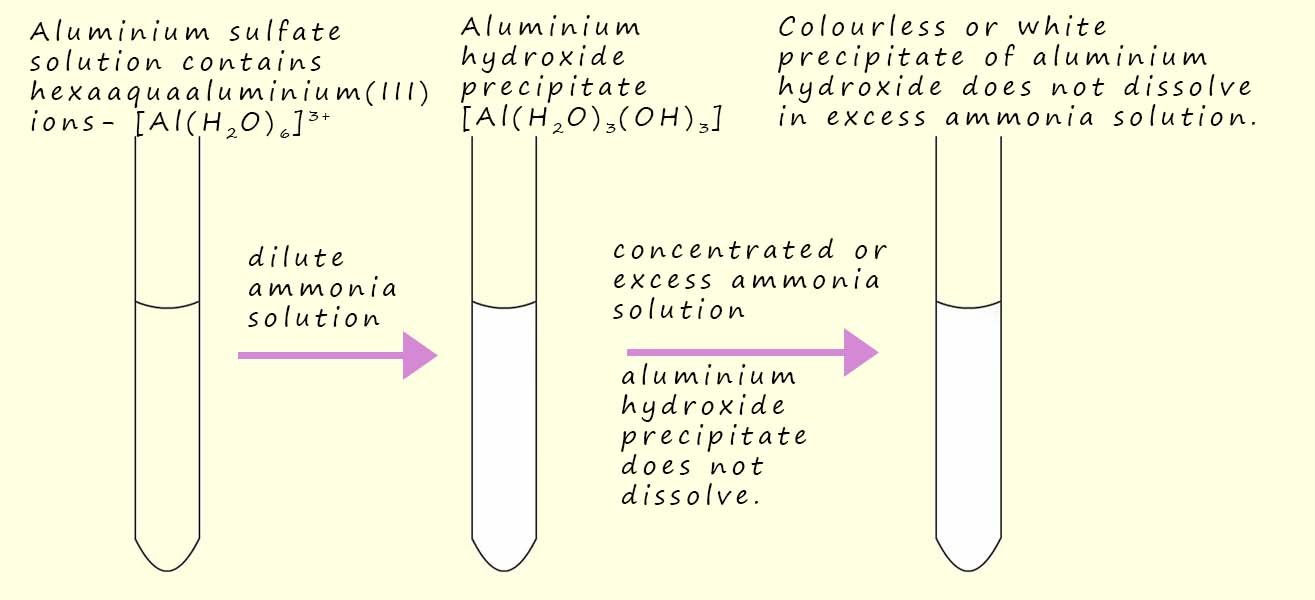
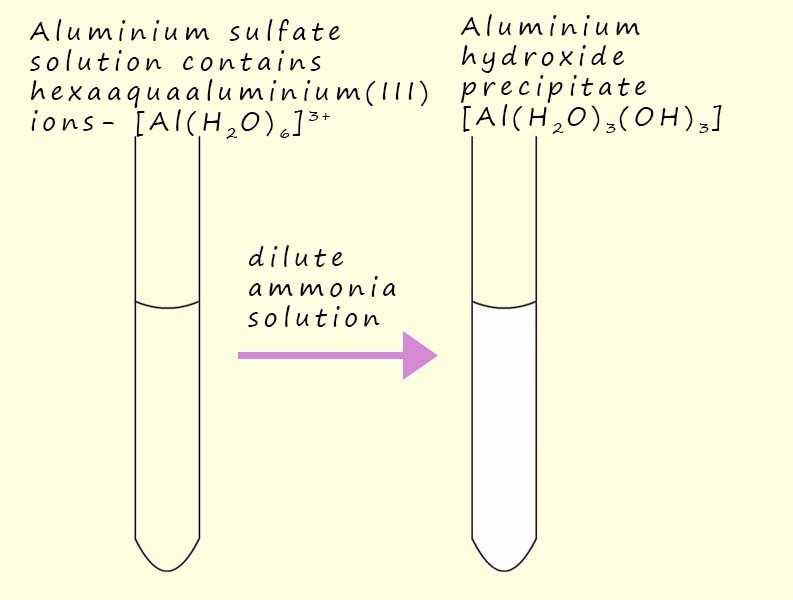 As an example consider the reaction that occurs when a dilute ammonia solution is added to a solution of aluminium sulfate -Al2(SO4)3. Addition of the dilute ammonia solution results in the formation of a colourless or white precipitate of aluminium hydroxide Al(OH)3(H2O). An equation and image to represent this reaction is shown below:
As an example consider the reaction that occurs when a dilute ammonia solution is added to a solution of aluminium sulfate -Al2(SO4)3. Addition of the dilute ammonia solution results in the formation of a colourless or white precipitate of aluminium hydroxide Al(OH)3(H2O). An equation and image to represent this reaction is shown below:
In the above reactions the ammonia is acting as a Brønsted-Lowry base, however the ammonia molecule can also use its lone pair of electrons to act as a Lewis base or a ligand, for example if a dilute solution of ammonia is added to a cobalt(II) chloride solution then as we have seen above a precipitate of cobalt hydroxide will be formed. However if an excess of ammonia or a concentrated solution of ammonia is added then the precipitate of cobalt hydroxide will dissolve and a pale brown solution is formed. This pale brown solution contains the hexaamminecobalt(II)complex- [Co(NH3)6]2+. This complex is formed as a result of a ligand substitution or exchange reaction. In this reaction the ammonia replaces the water as a ligand to form the hexaammine complex, we can show this ligand substitution reaction using the equation below, it is also highlighted in the image below:
It is important to keep the hexaamminecobalt(II) solution away from air since it is readily oxidised by the oxygen present in the air to form a reddish-brown solution containing the hexaamminecobalt(III) ion.
A similar ligand substitution reaction takes place with copper (II) ions (Cu2+). The blue precipitate of copper hydroxide that is formed when dilute ammonia is added to a copper sulfate solution will redissolve to form the deep blue solution containing the tetraammindiaaquacopper(II) - [Cu(NH3)4(H2O)2]2+ complex ion. This deep blue solution is formed as a result of four water ligands being replaced by ammonia or ammine ligands to form a stable complex, we can show this ligand substitution reaction using the equation below. The image below also summaries these reactions:
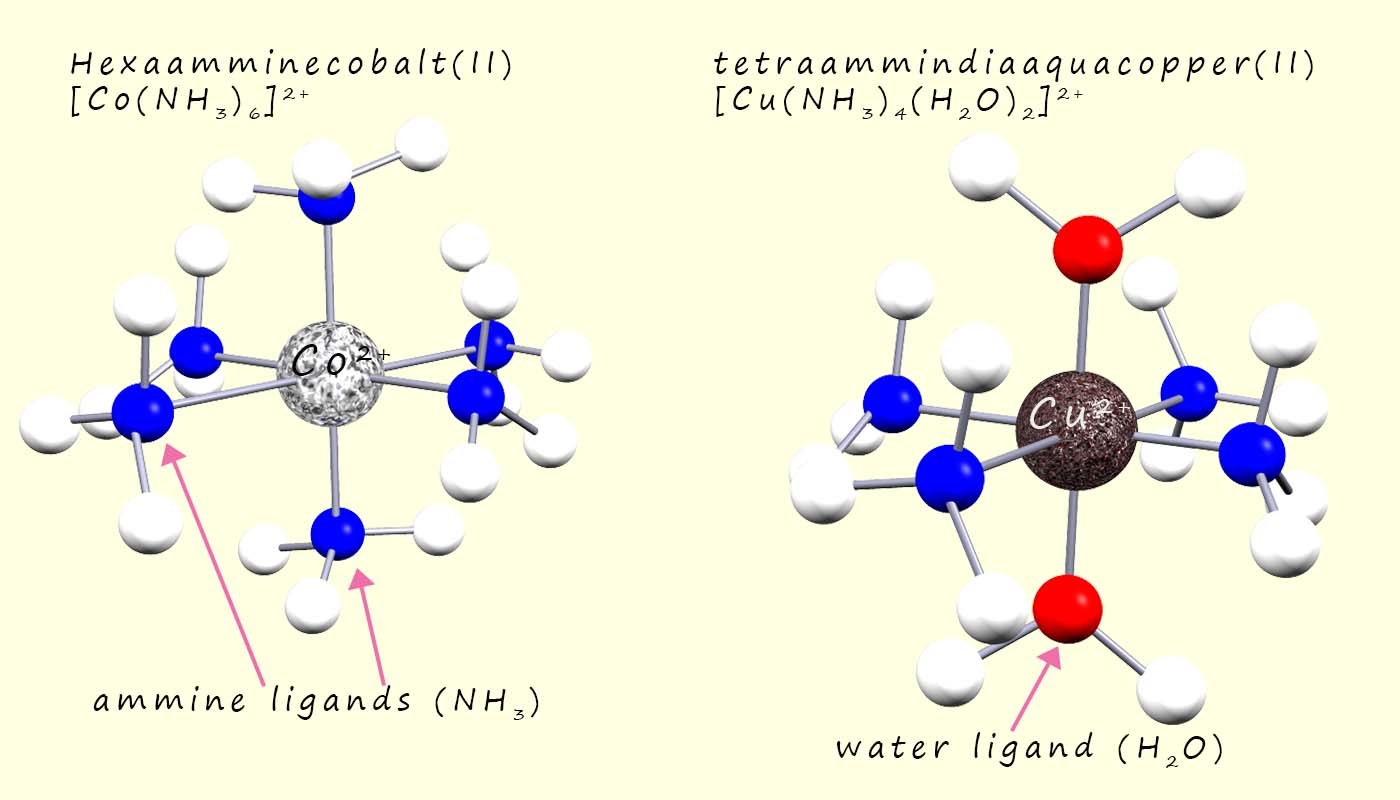
The hexaamminecobalt(II) and the tetraammindiaaquacopper(II) are both octahedral shaped complex ions, the cobalt(II) complex simply consists of six ammonia or ammine ligands surrounding the central cobalt ion. While in the tetraammindiaaquacopper(II) complex the water ligands occupy the axial positions while the ammonia ligands occupy the equatorial positions in the octahedral complex, this is outlined in the image below:
Not all insoluble metal hydroxides will undergo this ligand substitution reaction and redissolve to form stable complex ions. Aluminium hydroxide (Al(H2O)(OH)3) for example is a white insoluble solid that forms when dilute ammonia solution is added to a solution containing hexaaquaaluminium(III) ions -(Al(H2O)6)3+. The insoluble precipitate of aluminium hydroxide will NOT however this time redissolve if an excess of ammonia or a concentrated solution of ammonia is added, this is outlined in the image below.
To summarise we can say that when small amounts of dilute ammonia are added to dilute solutions of hexaaqua transition metal salts that the reaction will produce a precipitate of the metal hydroxide, however if a concentrated solution of ammonia is used or if an excess of ammonia solution is added then some of the metal hydroxide precipitates will redissolve as they undergo a ligand substitution or exchange reaction, though it is worth mentioning that not all metal hydroxides will undergo this ligand substitution reaction with concentrated ammonia solution. Some examples of the coloured precipitates that form when transition metal complexes have dilute ammonia solutions added are shown in the image below. The image also highlights some of the transition metal complexes that do not undergo further ligand substitution reactions when an excess or a concentrated ammonia solution is added.